Home > Events >Tofflon Interpretation: Development of Inspection for Lyophilized Product -Part 1: Introduction & History
Tofflon Interpretation: Development of Inspection for Lyophilized Product -Part 1: Introduction & History
Inspection and quality control of
parenteral products
There have been concerns about
contamination by particulate matter of injectable drugs since the development
and study of earliest intravenous treatments (1820’s and 1830’s).
The first requirement about visible
particles in injectable drugs for use in the United States is dated 1936 when
in the
As a matter of fact, all parenteral
products contain some level of particulate matter and this has the potential to
cause risk and harm to the receiving patient.
Even though this risk is very low on a
single dose/patient basis, if we keep into account that:
- millions of patients receiving
injectables
- usually a full treatment involves the
injection of many repeated doses
- health condition of receiving patients
can be already degraded
then the risk becomes significant and precautions must be taken to minimize it as much as possible.
What does it mean “parenteral”?
Basically, it’s any way of administration
different than oral, not going through the oral route.
Intravenous (IV), intramuscular (IM), and subcutaneous (SC) are the three most frequently used routes used as parenteral administration.
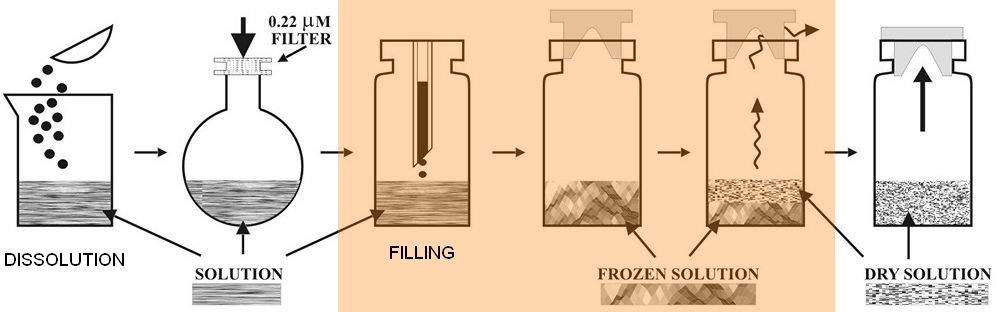
How are Lyophilized products affected?
In case of Lyophilized products, the issue
of unwanted particulate matter becomes trickier compared to liquid products
because of two main reasons:
1) The lyophilization process involves more
phases and longer time during which the containers are open or half-stoppered
and hence have a higher risk of contamination by foreign particles
2) Visual inspection of lyophilized products for particles is more difficult because final product is no more transparent and only the outer surface of the cake can be visually inspected.
What does it mean “particulate matter”?
Definition may change across different
times and Pharmacopeias, but we can use current USP description as a reference:
“mobile undissolved particles, other than gas bubbles, unintentionally present
in the solutions”
Patient risk
It is important to note that there isn’t
plenty of scientific data nor studies to support and assess the effects of
injection of extraneous particles to the human body. Obviously, the effects
greatly depend on the size, quantity, composition of particles on one side and
on patient’s clinical condition and injection way and location on the other and
it is not ethically acceptable to use patients as subjects for testing.
All data available today are therefore
either derived from studies on animal models or are derived from analysis of
cases where human subjects were accidentally injected with foreign bodies in
non-controlled situations (for example drug abusers).
Even if such available data are showing damages only in few cases and only when the number of foreign particles is massive, it has been widely and for a very long time accepted as a safety principle to take every possible action to avoid as much as possible the presence of foreign particles into all injectable products.

Which kind of inspection?
Different kind of inspection can be used to
verify the quality of containers and remove defective products.
The first basic distinction is between
destructive and non-destructive testing (DT / NDT).
Destructive Testing (DT) tests are carried out to the specimen's failure, in case of parenteral products this means opening the container and checking its content for sterility, contamination or alteration in laboratory.
Nondestructive testing (NDT) is a wide
group of analysis techniques used to evaluate the properties of a material,
component or system without causing damage. Because NDT does not permanently
alter the container being inspected, it is a highly valuable technique that can
save both money and time in product evaluation, troubleshooting, and research.
Finally, while DT procedures are generally specific to the industry and product
category, NDT techniques can often be used in different sectors and so are
developing with higher speed. For example, X-ray inspection developed initially
for aerospace industry is more and more used in pharmaceutical and food
industries too.
While DT can achieve much more precise and accurate results and it is sometimes the only way to measure some parameters (for example sterility), it is obviously impossible to perform DT on every product before its release.
Therefore, the inspection standard used today in pharmaceutical industry combines sampling destructive testing with 100% NDT techniques. This has become the inspection standard worldwide as IPQC (In Process Quality Control) tests for sterile dosage form and monitors the whole production process from raw material purchase and receiving, to their processing, manufacturing and packaging.
There are a number of quality controls to be performed on parenteral drugs during manufacturing (Leakage Test, Sterility Test, Pyrogen test, Clarity Test, pH, etc.).
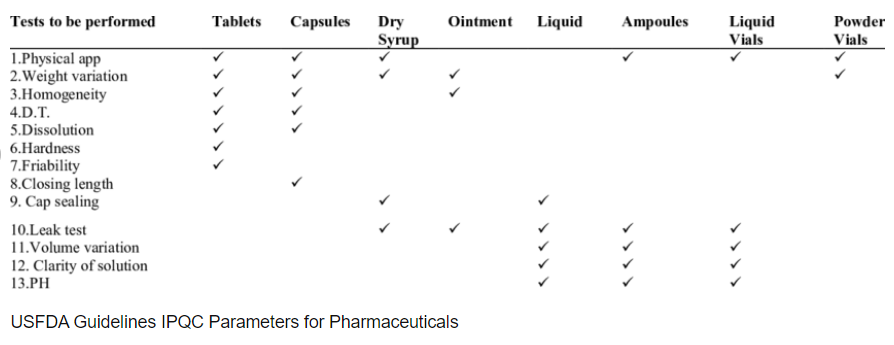
Most of them are out of the scope of this document which is focused on visual inspection.
“Visual” inspection
Historically quality inspection has always
been heavily based on vision, sight being the strongest, most powerful and
flexible sense we human being have and exploit to get information about the
world.
Interestingly, in ancient Greek language “I
know” (οἶδα, oida) was equivalent to “I have seen” so this simple fact shows
how already in the past the best way to know anything was to look carefully at
it first!
This basic idea is still the foundation of many inspection and controls today, with the important addition of machine vision that is basically mimicking human sight (eye+brain) with artificial components (camera+computer&software).

While on one side visual inspection still has several advantages (can be very quick and sensitive, it’s non-destructive, very flexible and adaptable to different controls on different objects) on the other hand it’s not always capable of detecting every defect as they’re hidden or “not visible” because of their nature or location, size, aspect, transparency.
“Non visual” inspection
To overcome the limits of visual inspection, in particular the visual inspection performed by human eyes and based on recent advancements in science& technology, electronics and computer science, more and more non-visual inspection systems have been developed and are gradually being introduced and used in all sectors of quality control.
- Non visual imaging (based on non-visible wavelength such as
X-rays radiography, Infrared thermography, laser transmission, etc.)
- Non optic imaging (Ultrasonic transmission and reflection
analysis)
- Reaction to high voltage / magnetic field analysis, eddy currently
- …